What is Dissolved Oxygen?
Dissolved Oxygen (DO) refers to molecular oxygen (O₂) that is dissolved in water. It differs from the oxygen atoms present in water molecules (H₂O), as it exists in water in the form of independent oxygen molecules, either originating from the atmosphere or generated through photosynthesis by aquatic plants. The concentration of DO is influenced by various factors, including temperature, salinity, water flow, and biological activities. As such, it serves as a critical indicator for assessing the health and pollution status of aquatic environments.
Dissolved oxygen plays a vital role in promoting microbial metabolism, influencing cellular respiration, growth, and the biosynthesis of metabolic products. However, higher levels of dissolved oxygen are not always beneficial. Excess oxygen may lead to further metabolism of accumulated products and potentially cause toxic reactions. The optimal DO levels vary among different bacterial species. For instance, during the biosynthesis of penicillin, DO is typically maintained at approximately 30% air saturation. If DO drops to zero and remains at that level for five minutes, product formation can be significantly impaired. If this condition persists for 20 minutes, irreversible damage may occur.
Currently, the most commonly used DO sensors can only measure relative air saturation, rather than the absolute concentration of dissolved oxygen. After sterilization of the culture medium, aeration and stirring are performed until the sensor reading stabilizes, at which point the value is set to 100% air saturation. Subsequent measurements during the fermentation process are based on this reference. Absolute DO values cannot be determined using standard sensors and require more advanced techniques, such as polarography. However, air saturation measurements are generally sufficient for monitoring and controlling fermentation processes.
Within a fermenter, DO levels can vary across different regions. Even when a stable reading is obtained at one point, fluctuations may still occur in certain culture media. Larger fermenters tend to exhibit greater spatial variations in DO levels, which can significantly affect microbial growth and productivity. Experimental evidence has shown that, although the average DO level may be 30%, fermentation performance under fluctuating conditions is notably lower than under stable conditions. Therefore, in the scale-up of fermenters—beyond considerations of geometric and power similarity—minimizing spatial DO variations remains a key research objective.
Why is Dissolved Oxygen Monitoring Essential in Biopharmaceutical Fermentation?
1. To Maintain the Optimal Growth Environment for Microorganisms or Cells
Industrial fermentation typically involves aerobic microorganisms, such as Escherichia coli and yeast, or mammalian cells, such as Chinese Hamster Ovary (CHO) cells. These cells function as "workers" within the fermentation system, requiring oxygen for respiration and metabolic activity. Oxygen serves as the terminal electron acceptor in aerobic respiration, enabling the production of energy in the form of ATP. Insufficient oxygen supply can lead to cellular suffocation, growth arrest, or even cell death, ultimately resulting in fermentation failure. Monitoring DO levels ensures that oxygen concentrations remain within the optimal range for sustained cell growth and viability.
2. To Ensure Efficient Synthesis of Target Products
The objective of biopharmaceutical fermentation is not merely to promote cell proliferation but to facilitate the efficient synthesis of desired target products, such as insulin, monoclonal antibodies, vaccines, and enzymes. These biosynthetic pathways often require substantial energy input, primarily derived from aerobic respiration. Additionally, many enzymatic systems involved in product synthesis directly depend on oxygen. Oxygen deficiency may disrupt or reduce the efficiency of these pathways.
Moreover, DO levels act as a regulatory signal. Both excessively high and low DO concentrations can:
- Alter cellular metabolic pathways, for example, shifting from aerobic respiration to less efficient anaerobic fermentation.
- Trigger cellular stress responses, leading to the production of undesirable by-products.
- Influence the expression levels of exogenous proteins.
By precisely controlling DO levels at different stages of fermentation, it is possible to guide cellular metabolism toward maximal target product synthesis, thereby achieving high-density and high-yield fermentation.
3. To Prevent Oxygen Deficiency or Excess
Oxygen deficiency (hypoxia) can have severe consequences:
- Cell growth and product synthesis cease.
- Metabolism shifts to anaerobic pathways, resulting in the accumulation of organic acids such as lactic acid and acetic acid, which lower the pH of the culture medium and may poison the cells.
- Prolonged hypoxia can cause irreversible damage, with recovery being incomplete even after oxygen supply is restored.
Excess oxygen (supersaturation) also poses risks:
- It can induce oxidative stress and the formation of reactive oxygen species (ROS), which damage cell membranes and biomolecules.
- Excessive aeration and agitation increase energy consumption and operational costs, leading to unnecessary resource waste.
4. As a Critical Parameter for Real-Time Monitoring and Feedback Control
DO is a real-time, continuous, and comprehensive parameter that reflects the internal conditions of the fermentation system. Changes in DO levels can sensitively indicate various physiological and operational states:
- Rapid cell growth increases oxygen consumption, causing DO levels to decline.
- Substrate depletion or inhibition slows metabolism, reducing oxygen consumption and causing DO levels to rise.
- Contamination by foreign microorganisms alters the oxygen consumption pattern, leading to abnormal DO fluctuations and serving as an early warning signal.
- Equipment malfunctions, such as stirrer failure, ventilation pipe blockage, or filter fouling, can also result in abnormal DO behavior.
By integrating real-time DO monitoring into an automated feedback control system, precise regulation of DO levels can be achieved through dynamic adjustments of the following parameters:
- Stirring speed: Increasing the speed enhances gas-liquid contact by breaking up bubbles, thereby improving oxygen transfer efficiency. This is the most commonly used and effective method.
- Aeration rate: Adjusting the flow rate or composition of the inlet gas (e.g., increasing the proportion of air or pure oxygen).
- Tank pressure: Elevating pressure increases oxygen partial pressure, thereby enhancing solubility.
- Temperature: Lowering the temperature increases oxygen solubility in the culture medium.
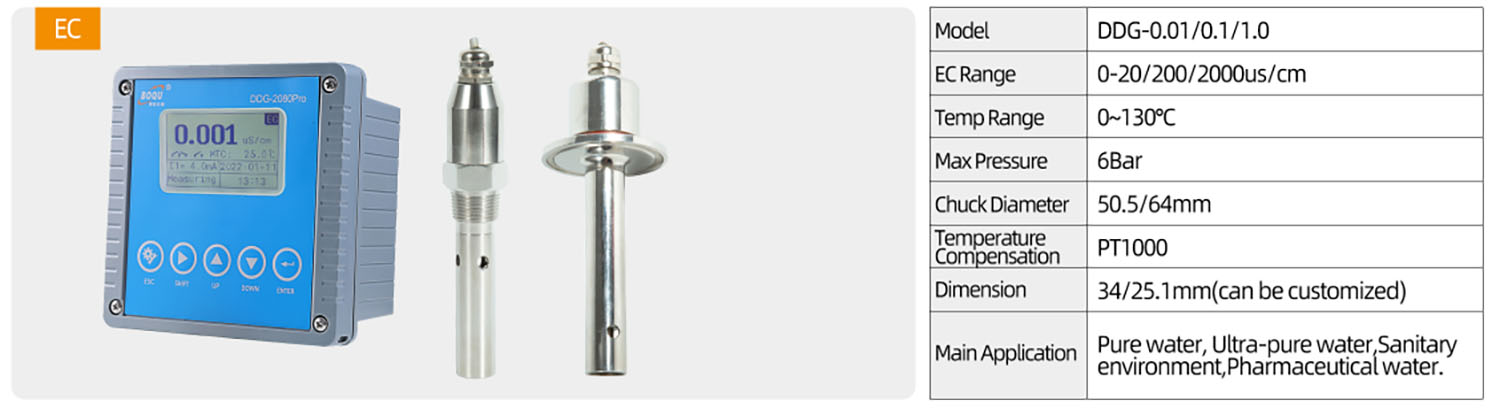
BOQU's product recommendations for online monitoring of biological fermentation:
Post time: Sep-16-2025